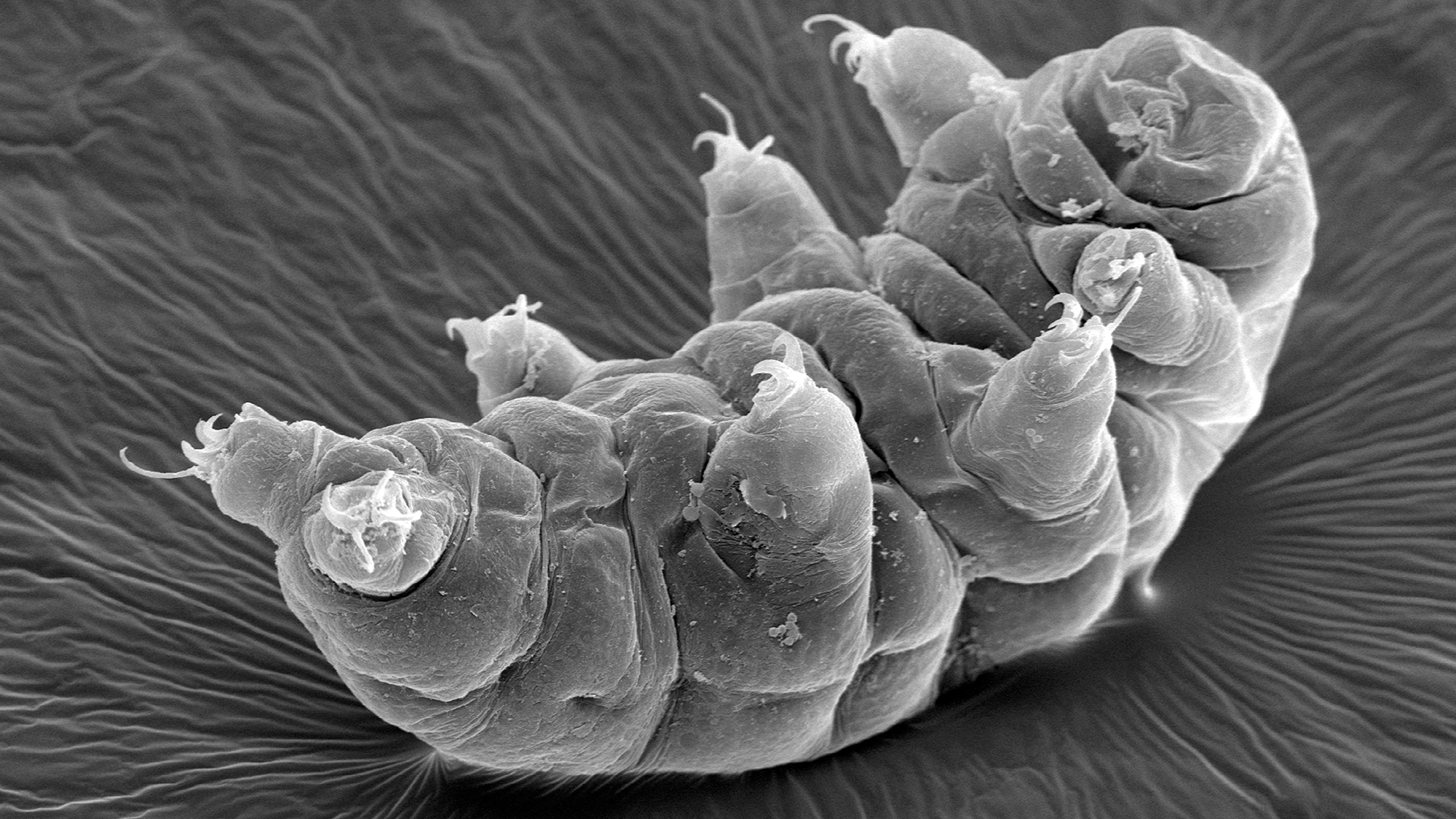
Apart from their disconcerting cuteness, tardigrades are famed for the fact that they’re nigh on indestructible. These tiny creatures are extremophiles, adapted for life in environments that would kill most other organisms. Different species demonstrate different resistances, and one of the most intriguing is some species’ ability to survive huge amounts of ionizing radiation—in some cases, over 1,000 times the dose that would be required to kill a human.
Earlier this year, scientists examined a species of tardigrade called Hypsibius exemplaris in the hope of understanding how it can withstand such huge doses of radiation. To their surprise, they found that the tardigrade hadn’t evolved some sort of way to shield its DNA from the damage caused by this radiation; instead, they showed a remarkable ability to repair that damage quickly and efficiently.
[Related: How super resilient tardigrades can fix their radiation-damaged DNA]
A new study, published October 24 in Science, investigates the radiotolerance of another species of tardigrade, Hypsibius henanensis. (This is a new species, discovered by the researchers themselves and named in honor of the Chinese province of Henan.) These tardigrades were exposed to heavy doses of gamma rays—high-energy photons that are one form of ionizing radiation—and researchers then studied how their systems responded.
They found that while H. henanensis demonstrates the same ability to repair damaged DNA as H. exemplaris, it also has a couple of other tricks up its sleeve.
The danger that ionizing radiation poses to living creatures comes from its ability to ionize atoms: a gamma ray photon carries enough energy that when it strikes an atom, it can essentially knock one of that atom’s electrons loose. If that atom happens to be part of a DNA molecule, the result can be a breakage in one or both of the delicate strands that wind into the famous double helix. Breakages of both strands are particularly dangerous, but any damage can prevent the molecule from replicating properly. (This is how ionizing radiation can cause cancer.)
When H. henanensis is exposed to radiation, three key mechanisms spring into action. The first is the same turbocharged DNA repair capability demonstrated by H. exemplaris. This is driven by a protein called TRID1, which appears to be unique to tardigrades. The researchers behind the new study examined exactly how TRID1 responds to radiation exposure. They found that it collects at sites where DNA has suffered double-strand breakages, encouraging the accumulation of another protein called 53BPI, which appears to be critical to the repair of double-strand breakages.
Researchers also noted a gene that springs into action in response to radiation exposure. This gene, BSC1, responds to radiation by up-regulating the production of two proteins known to be important for mitochondrial synthesis of ATP. The fact that H. henanensis produces them en masse in response to radiation exposure led researchers to theorize they may also play a role in repairing DNA. This may also explain why radiation exposure in humans can result in malfunctioning mitochondria. “Our study showed an unexpected link between mitochondrial proteins and nuclear DNA repair, providing an optional explanation for mitochondrial dysregulation after radiation exposure.”
H. henanensis also appears to be able to minimize the amount of damage done by ionizing radiation in the first place. While direct damage to DNA can be catastrophic, ionizing radiation can also cause damage in other ways. As the paper explains, “[Ionizing radiation] exerts its biological effects through two mechanisms: direct action and indirect action. The latter, mediated by reactive oxygen species (ROS), accounts for 60% to 70% of the [radiation’s] effects of IR.”
Life has its own defense system against free radicals, in the form of antioxidants. These molecules react with free radicals, effectively neutralizing them, and there’s generally a balance of both in living organisms. However, when there are so many free radicals in an organism’s system that its antioxidant capabilities are overloaded, the organism experiences “oxidative stress”—a state where the ROS molecules are free to react with cellular tissue, DNA molecules, and anything else unfortunate enough to be in their way.
H. henanensis deals with this problem by producing large quantities of proteins called betalains in response to radiation exposure. These proteins are highly effective antioxidants, and they work to essentially mop up the excess free radicals before they can wreak havoc on the tardigrade’s system. The presence of betalains in tardigrades is notable because they are usually found in plants. Their production is regulated by a gene called DODA1, which the researchers speculate arrived in tardigrades via horizontal gene transfer, most likely from a bacterium in the phylum Bdellovibrionota.As well as being fascinating in and of itself, understanding how tardigrades survive radiation exposure could also help humans do the same. Earlier this year, inspired by the research into H. exemplaris, researchers found that the introduction of TRID1 into human cells seemed to boost those cells’ ability to resist DNA damage. Given that there is still a great deal we don’t understand about tardigrades’ resilience, these humble little animals might yet have many more secrets to reveal. As the team behind this new paper said in a statement, “Extremophiles such as tardigrades [are] a treasure trove of unexplored molecular mechanisms of stress resistance. Functional research on these radiotolerance mechanisms… will further broaden our understanding of cellular survival under extreme conditions and may provide inspiration for promoting human health and combating disease.”